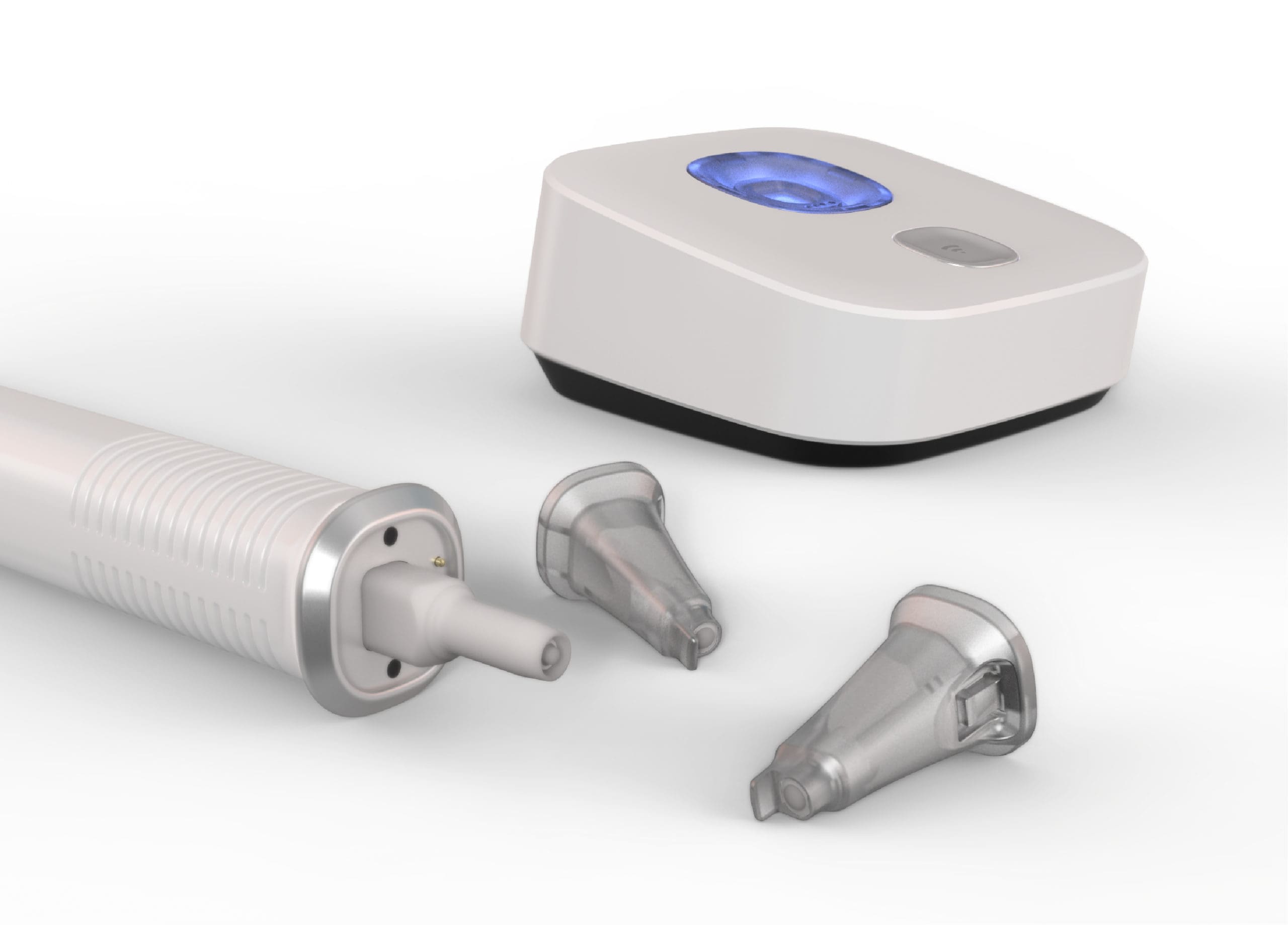
Medical device design and development requires an understanding of customer and user needs, healthcare regulatory issues, and the design for manufacturing and engineering processes. This post is the first in a series that begins a comprehensive guide for Medtech professionals to understand how medical devices are designed, engineered, and developed. Part 2, “How to Design a Medical Device” is coming soon and goes into our step-by-step methodology for designing, engineering, and developing medical devices.

Who designs medical devices?
Some considerations on who to look for
When designing a medical device, an initial consideration is whether to design, engineer and develop in house or with a design and engineering firm. Of the three broad variations of this choice, in-house, particularly in-house and external, have additional choices and tradeoffs.
A company might choose to design a medical device in-house for any number of reasons. Many companies have existing resources such as product designers, engineers, and development engineers. These companies may also have expert knowledge in a particular type of device or medical environment. However, without existing dedicated resources, a company should struggle to justify creating resources needed to design and or develop a new medical product. In fact, despite their previous experience, a newly created team would be at a disadvantage to an existing external team that has years of collaborative work together designing and developing devices.
Companies looking to move fast and focus on the commercialization of a medical device and its technology often choose an external team to augment or completely replace an internal team. Not all design firms have engineering capabilities, so a company needs to understand where they need assistance. In practice, working with multiple firms on a project can lead to delays. Therefore, finding a firm that does both design and engineering can increase the process’ efficiency if a company is looking for development. Overall, an external team with medical design experience, engineering, and development can move more quickly than an internal team due to finely-tuned processes. Most design and engineering firms work on projects in the consumer, industrial, and durable markets. This experience can often be useful for the design, engineering, and development of a medical device. Cross-pollination of ideas can lead to more innovative and more human-centric devices, and more likely to achieve broad adoption in the marketplace.

Who develops medical devices?
If you’re developing medical devices, you probably have an existing foundation in the space or are a medical product company already. It’s normal for a company to do extensive research of a problem and technology before engaging their own designer and engineers or an outside firm to develop a medical device. Juxtaposed against the entrepreneurial archetype single-handedly innovating a new product or category, medical device entrepreneurs are often technology experts or established firms. Usually, medical devices require a support network of innovation and expertise in the space. Medical companies that want to innovate are the primary driver of new medical devices. They usually have core functionality in one area and are looking for a way to operationalize that expertise in a new product or slightly expand that expertise. Often the product will be targeted for a category that will grow quickly, which usually means combining a couple of products functionalities into one device.
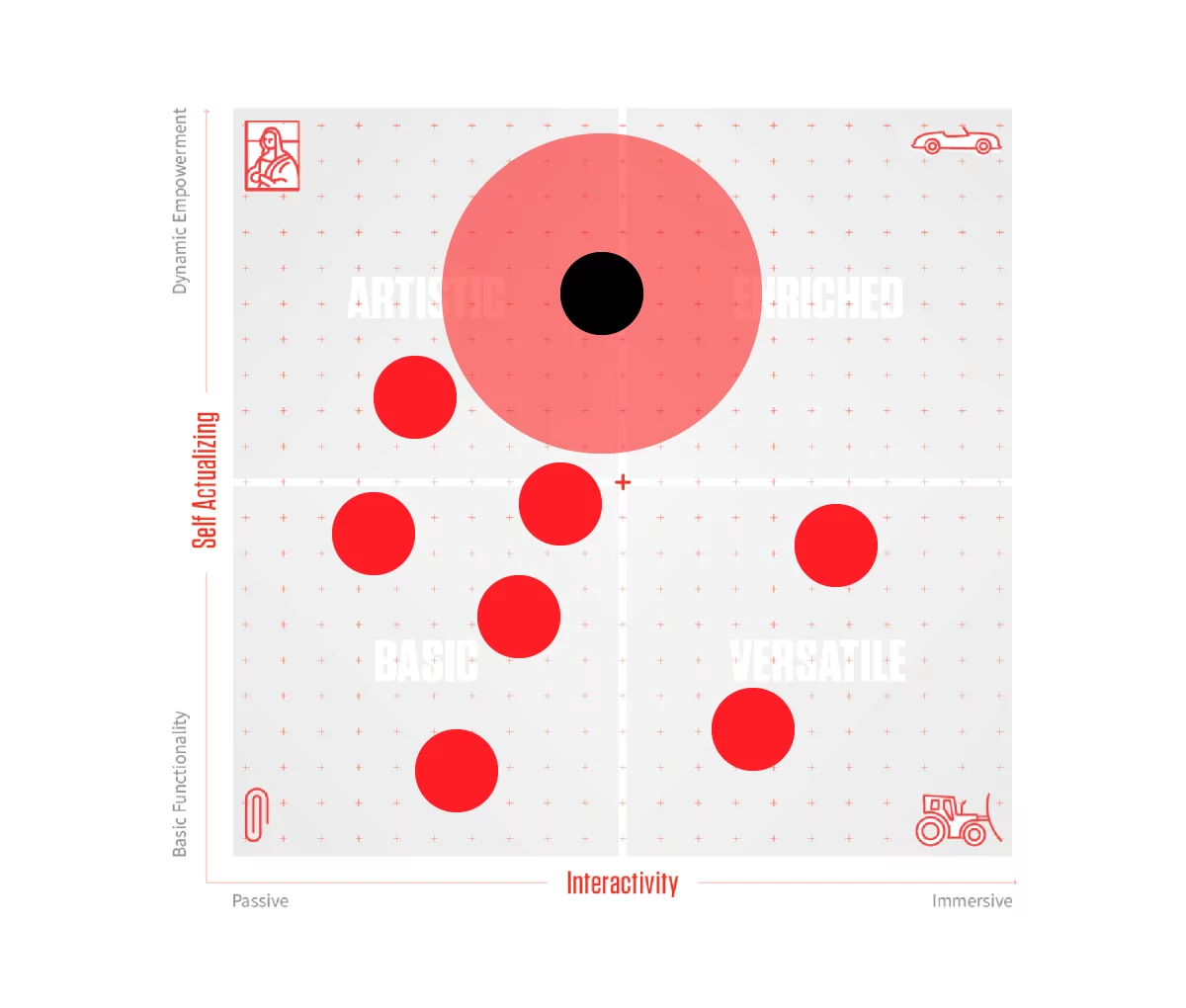
What is the difference between consumer products and medical devices?
While some of the best medical device designs can be mistaken for consumer devices, the design, development, and engineering process between them is drastically different. The level of performance and functionality required from medical devices is much different. Whereas human factors and usability issues in a consumer product might be inconvenient, they are mission-critical for medical devices, where user or device error can lead to life-threatening complications. In addition, regulatory issues around materials choices and technology are both complex and core to a medical device’s success. Medical devices often have to be approved for use, and failure to gain approval can be a devastating result for a multi-year long design, engineering, and development process. This means that newer technologies that can be used in consumer products often can’t be used in medical devices until several years later. Medical devices are often limited by both the available biocompatible materials and approved technologies. Nevertheless, there has been a trend to design medical products more like consumer products. Indeed, properly designed and engineered devices can achieve broad market success similar to consumer products.